Rotation Projects 2024-2025
Welcome to the University of Pittsburgh School of Medicine Interdisciplinary Biomedical Graduate Program. Rotation opportunities available in the Cell Biology and Molecular Physiology (CBMP) Graduate Program are listed below. Faculty are drawn from both basic science and clinical departments. Research in the CBMP program is focused on normal cellular biology and function, as well as disfunction in renal, liver, lung, eye and heart disease, cancer, diabetes, ageing, and inherited disorders of developmental and reproductive functions. If you are interested in a rotation opportunity, speak to faculty at orientation or contact them by email.
- Lipid signaling in healthy cells and cancer - Gerry Hammond
Cancers are caused by mutations that drive uncontrolled growth and proliferation ofcells, and eventually unbridled migration. A common class of mutations is found in more than half of all cancer cases: those that activate a central lipid signalingpathway – the PI 3-kinase or PI3K pathway. The lipid products of this pathway activate downstream cellular programs promoting proliferation, survival and migration – all hallmarks of tumors.
So it seems like a good idea to stop this pathway with PI3K inhibitors, and big pharma has many compounds approved or in late-stage clinical development.The huge caveat is that PI3K is crucial for other processes in the body – such as activation of the immune system, and the cellular response to insulin. So, PI3K blockers are not well tolerated. We need a more nuanced approach.
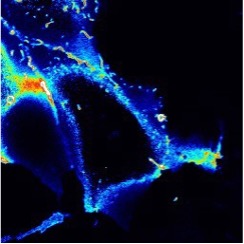
We are studying the lipid products of PI3K. Whilst the pathway is famous, it is less well known that the pathway actually produces two signaling lipids – a major and minor lipid. We developed a biosensor for the latter (the rainbow colored image, above). We have also used gene editing to tag the enzymes involvedso we can track their activity in intact cells. While no one really understood what this lipid is doing, we can now study it in real time.
The aim of the project is to test the hypothesis that this minor lipid actually drives long term proliferative signaling intumors, whilst the major lipid is responsible for more acute effects of insulin or activation of the immune system. We therefore believe that down-regulating the minor lipid will selectively attenuate signaling in tumors, whilst sparing normalimmunological and metabolic pathways elsewhere in the body. As an added bonus, this may actually improve insulin sensitivity and reduce the accumulation of tauopathy (as seen in Alzheimer’s disease).
The project will involve training in live cell imaging, molecular genetics and gene editing in cell culture models.
Learn more at: hammondlab.com
Contact Dr. Hammond.
- Integrative Single-Cell and Spatial Transcriptomic Profiling of Cardiac Development and Disease - Guang Li
The Li lab uses human induced pluripotent stem cell (hiPSC)-derived heart organoids and mice as primary research models. Recently, the lab generated a comprehensive single-cell RNA sequencing dataset encompassing 18 developmental stages of mouse hearts during embryonic and neonatal periods. This dataset, published in Nature Communications, represents a valuable resource for the field. The lab has also analyzed the heterogeneity and function of cardiac fibroblasts at different stages of mouse heart development, with the results published in eLife. Moreover, the lab has generated an atlas of mouse spatial transcriptomic data using sequencing-based approaches, including Slide-seq and Visium. This work is currently under revision at Nature Communications. Building on these studies, the lab currently is focusing on the generation of large imaging datasets including immunofluorescence staining, single-cell RNA-seq, and spatial transcriptomics images, to train deep learning models for analyzing cardiac cell states under normal and disease conditions.
The lab also reported the generation of atrial and ventricular heart organoids, which were used to model chamber deficiencies in a hiPSC line carrying a point mutation in the Nkx2-5 gene. This study was published in Communications Biology. More recently, the lab has further improved the organoid model by inducing additional cardiac cell types, such as endothelial and epicardial cells. The lab has also developed multi-chambered organoids with detailed structures, including valves. The long-term goal of the lab is to generate 4-chambered heart organoids to model disease progression.
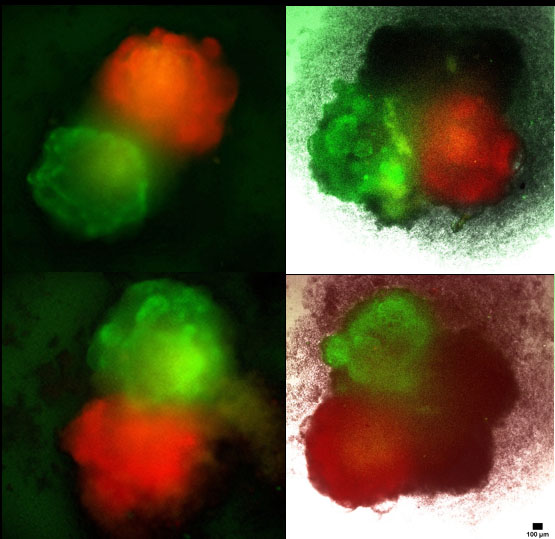
Lab: Li Lab
Contact Dr. Li
- Advancing Safe and Effective Therapeutic Delivery for Hearing Loss - Adele Moatti
Our mission is to help safely, reliably, and effectively deliver therapeutics useful for the clinical treatment of hearing loss. Our lab addresses the significant challenges inherent in developing effective inner ear substance delivery systems. One key hurdle involves not knowing the exact drug formulation for which the delivery system is being designed, affecting drug bioavailability and biodistribution. Another obstacle lies in translating promising findings from preclinical studies, conducted on animal models, into applicable solutions for human patients.
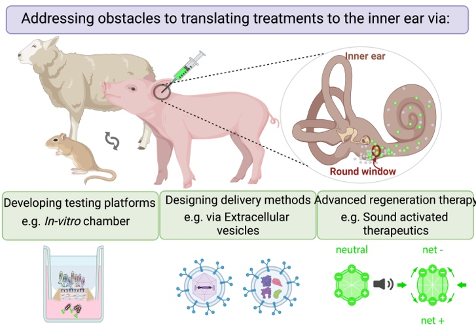
Lab: Moatti Lab
Contact Dr. Moatti
- Pathogenesis and Therapeutic Targeting of Acute Kidney Injury - Sunny Sims-Lucas
Acute kidney injury (AKI) occurs in nearly 1 of 5 hospitalized patients and is associated with increased morbidity and mortality across all ages. Many AKI patients will recover kidney function post-injury but then progress to chronic kidney disease (CKD). The mechanisms are poorly understood and there are currently no effective therapies to prevent, limit, or reverse the tissue damage. There is a critical need to identify mechanisms involved in the pathogenesis of AKI. Our long-term goal is to elucidate these mechanisms and leverage them for new therapies to limit AKI and prevent the transition to CKD. We have three major projects that are currently be investigated:
1. Sirtuin 5 mediated protection against AKI.
2. Role of succinylation in kidney injury
3. Diacarboxylic acids as a therapeutic for AKI and the progression to CKD.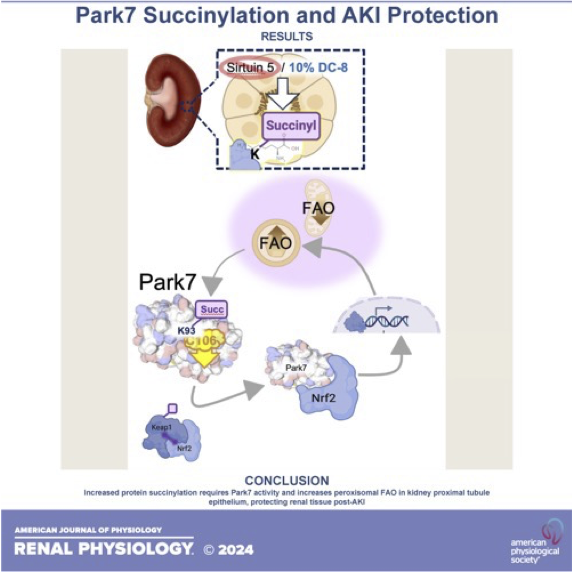
Contact Dr. Sims-Lucas.
- Wnk signaling from biomolecular condensates - Arohan Subramanya
Our laboratory studies a kinase signaling pathway that forms biomolecular condensates – membraneless droplet-like assemblies that form via a process called phase separation. During cell shrinkage caused by hyperosmotic stress, water exits the cell, resulting in the crowding of cytoplasmic contents. In a recent paper (Boyd-Shiwarski et al, Cell 2022), we showed that WNK kinases sense this crowding, forming phase separated condensates that activate a phosphorylation-dependent cell volume recovery signal. While the ancient and ubiquitous version of this cascade regulates fluid volume in cells throughout the body, kidney tubules uniquely leverage the crowding-induced signal to control salt and water reabsorption, blood pressure, and potassium balance. Thus, WNK kinases provide one of the first clear examples of a signaling pathway that forms biomolecular condensates to control cell and whole animal physiology.
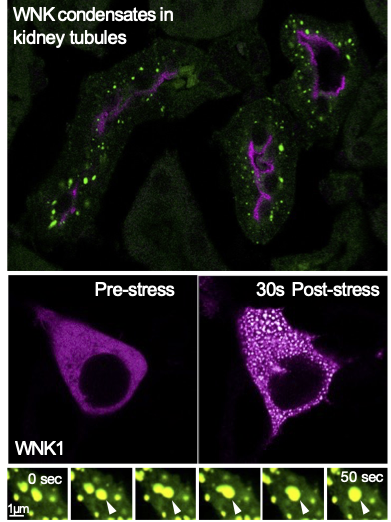
This discovery has opened several avenues of investigation that could be potential rotation projects. These include (1) determining the mechanism by which WNK kinases activate signaling within condensates to drive cell volume rescue, (2) elucidating signatures within the kinase that function as crowding-induced phase separation drivers, (3) identifying novel signaling partners that reside within WNK droplets, (4) testing the relevance of phase
separation in physiology using established mouse models of kidney-specific WNK condensate hyperactivation and dysfunction, and (5) testing how recently identified small molecule inhibitors of the WNK signaling pathway influence its stress-induced phase behavior.Learn more at http://www.subramanyalab.org.
Contact Dr. Subramanya
- Cytoskeleton-driven motility in invasive parasites- Stella Sun
The Sun Lab is interested to attain an in-depth, molecular and mechanistic understanding of how cytoskeleton-driven cell motility operates in highly invasive human parasites responsible for significant infectious diseases. Our research focuses on flagellate eukaryotic parasites,
specifically Trypanosoma brucei (T. brucei), lead to the lethal disease Trypanosomiasis. The swimming ability of these parasites is facilitated by a sensory organelle called the flagellum, which serves as an organelle ruler for cell size. This single flagellum connect to the cell body through membrane- membrane junctions, affecting the parasite’s size, deformability, and swimming behavior.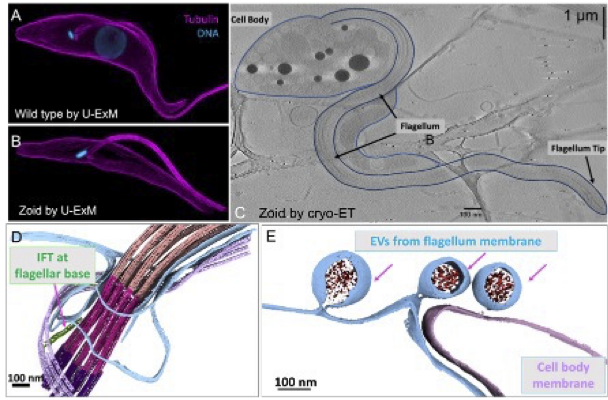
Our goal is to utilize a genetically engineered miniature T. brucei cell system called zoid and a cutting edge cryo-EM strategy spanning multiple scale to unravel the intricate process by which the intraflagellar transport (IFT) mechanism for flagellum assembly and maintains. Additionally, we seek to elucidate how extracellular vesicles (EVs), a process vital for cell-cell communication, can be coupled with flagellum membrane remodeling. By establishing this imaging platform, the researchers aim to contribute to the broader cryo-electron microscopy community’s knowledge and applications in cell biology studies, particularly those related to infectious diseases.
Potential rotation projects include:
1) Unraveling the gating mechanism of IFT within the flagellum base.
2) Exploring the assembly and function of EVs in cell-cell communication.
3) Investigating the role of the essential organelle biogenesis such as close mitosis.For more information on the Sun lab, see: https://sunlab.structbio.pitt.edu
Contact Dr. Sun- Cellular principles of aging and age-related disease - Jay Tan
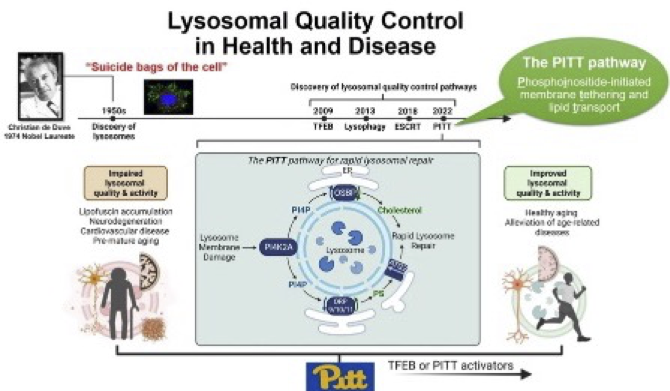
The Tan lab studies molecular and biochemical mechanisms of cellular quality control in aging and age-related disease. We search for essential, unifying molecular principles behind complex stress responses using unbiased approaches, and dissect the underlying mechanisms using multidisciplinary methods including molecular biology, biochemistry, cell biology, genetics, proteomics, bioinformatics, and so on. We are actively working on two broad directions. (1) Lysosomal quality control mechanisms in response to cellular stress stimuli: Lysosomes are known as the longevity-promoting organelles, the dysfunction of which is commonly found in aging and age-related diseases including all kinds of dementia. We are searching for universal mechanisms underlying lysosomal quality control in response to diverse age-related cellular stresses, with the goal of discovering new strategies to promote longevity through lysosomal rejuvenation. Our recent discoveries in this direction include an essential rapid lysosomal repair pathway (the PITT pathway). (2) Innate immune signaling in aging and neurodegeneration. Abnormal exposure of DNA fragments in the cytosol is a common condition during microbial infection, cell damage, organismal aging, cellular senescence, and degenerative diseases. Such DNA exposure triggers the cGAS/STING DNA-sensing innate immune pathway to protect cells from infection but causes pathological consequences in other contexts. Our research focuses on elucidating the molecular mechanisms that underlie the detrimental functions of this pathway, including examining its interactions with lysosomes. Our goal is to develop targeted interventions that can inhibit its pathogenic effects while preserving its antimicrobial activity. Our recent progress in this direction includes a conserved ion channel function of STING that mediates noncanonical autophagy and cell death.
Rotation projects include, but not limited to:
a. Biochemical screening of lysosomal stress response pathways using established approaches in the lab.
b. Cell biological screens of membrane contacts in cellular stress response using established tools.
c. Examine the impact of the PITT pathway on the aggregate clearance activity of microglia.More information can be found at: JayTanLab.org
Contact Dr. Tan
- mRNA mRNA translation regulation in cell migration - Deepika Vasudevan
Lab overview: There are several active lines of investigation in my lab that are mutually independent and thematically converge on understanding the role of stress response signaling in disease and development. Projects in the lab are directed at uncovering new mechanisms of mRNA translation regulation during cellular stress (e.g. PMID32938929), the phenotypic consequences of disrupting stress response signaling in metabolic tissues (e.g. PMID 38457339), and the role of these signaling pathways in visual system development and disease (e.g. PMID 34919148). The lab uses a potent combination of Drosophila (fruit fly) model, cultured human cells, and classic biochemistry approaches to comprehensively map the molecular mechanisms and physiological relevance of stress response signaling.
Rotation/thesis project: We recently discovered that loss of the stress response transcription factor, ATF4, results in cell migration defects using the Drosophila ovary as a model (see figure showing live imaging of migrating ‘border’ cells in green making their way through germline cells in red). Though ATF4 is a well-studied transcription factor, its role in collective cell migration is unknown. This is worth investigating particularly because ATF4 and associated stress response pathway components are frequently upregulated in many cancers. Open questions include:
1) How are ATF4 levels regulated in migrating cells?
2) What are the binding partners of ATF4 in migrating cells?
3) What are the transcriptional targets of ATF4 that effect migration?The project(s) will use a potent combination of Drosophila genetics, live imaging, and cell culture experiments to examine the above questions.
Contact Dr. Vasudevan
- Ubiquilins and Alzheimer’s Disease and Related Dementias - Matt Wohlever
A hallmark of Alzheimer’s Disease and Related Dementias (ADRDs) is defects in proteostasis. Ubiquilins are a family of proteins that serve as a major hub in the proteostasis network and are also intimately linked with ADRDs. For example, single point mutations in Ubiquilins lead to Amyotrophic Lateral Sclerosis (ALS). Despite the clear medical relevance, the underlying mechanisms of Ubiquilin function are not understood at the molecular, subcellular, and neuronal levels, which presents a significant barrier to developing targeted therapeutics to treat ADRDs. Paradoxically, while Ubiquilins are traditionally thought to shuttle proteins to the proteasome for degradation, there are many reports in the literature of Ubiquilins stabilizing client proteins, including many ADRD proteins. It is unclear how this triage decision is made and executed.
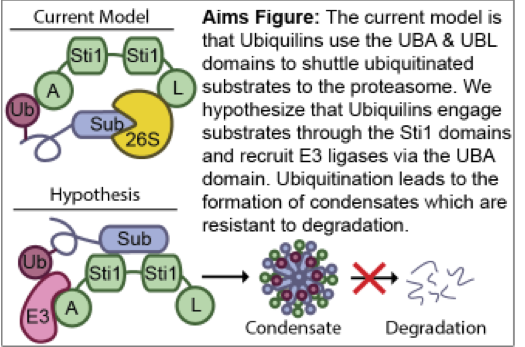
We have recently discovered that Ubiquilins directly bind to ADRD proteins via the Sti1 domain and recruit E3 ligases to promote the formation of biomolecular condensates that protect substrates from degradation (Fig 1). This contrasts with the existing model, which posits that Ubiquilins interact with ubiquitinated proteins via the UBA domain and then shuttles these proteins to the proteasome for degradation. The new paradigm of Ubiquilins as molecular chaperones that stabilize ADRD proteins via the formation of condensates will fundamentally shift our approach for developing therapeutic interventions in Alzheimer’s Disease and Related Dementias.
https://wohleverlab.wordpress.com/lab-members/matt-wohlever/Contact Dr. Wohlever
- Unlocking the secretes of proteostasis: does the 12h oscillator hold the key for treatment of neurodegeneration - Bokai Zhu
Proteostasis, the cellular mechanism that governs the synthesis, folding, and degradation of proteins, is crucial for maintaining proteome integrity, especially under proteotoxic stress. A decline in protein quality control is closely linked to aging and neurodegenerative diseases. Traditionally, proteostasis was believed to be constant under basal conditions.
However, using novel mathematical tools, our lab has uncovered a surprising 12-hour rhythm in global proteostasis dynamics in normal cells, operating under physiological conditions. This 12-hour rhythm is regulated by a cell-autonomous mammalian 12-hour ultradian oscillator, independent of the 24-hour circadian clock and the cell cycle. Our lab is interested in studying the gene regulatory network, chromatin and epigenetic landscape, biological function, and evolutionary origin of the 12-hour oscillator. We are further pursuing a new direction following our recent discovery of the critical role of nuclear speckle liquid-liquid phase separation in controlling proteostasis. We are actively studying how nuclear speckle sense proteostasis stress and preemptively elicit stress response. Lastly, we are developing innovative strategies for rejuvenating nuclear speckles to combat protein misfolding diseases, such as tauopathy in neurodegeneration.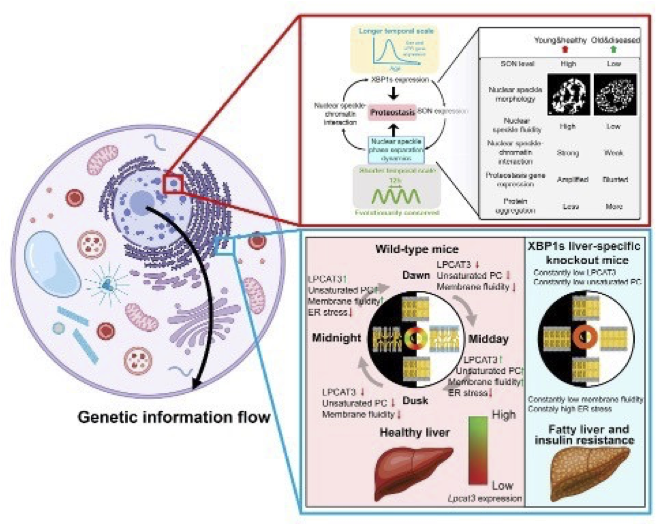
Potential rotation projects:
1) Developing 12h-clock reporter system using CRISPR-CAS9 based imaging approaches
2) Identifying non-cell autonomous regulators of 12h clock and protein quality control using innovative proximity- labeling biochemical approaches
3) Identify epigenetic regulators of mammalian 12h clock and UPR.
4) 12h clock in senescence and aging.
5) 12h clock in humans.
6) Nuclear speckle rejuvenation in combating proteinopathies.
Bokai website link: www.bzhulab.comContact Dr. Zhu
